The use of TruScient® is supported by evidence from a randomised, controlled, multi-centre trial in 133 dogs.
The study included dogs with acute diaphyseal fracture of a long bone (femur, humerus, radius, radius + ulna, ulna, tibia, tibia + fibula).
Intervention consisted of either surgical standard of care (SOC) alone or TruScient® in combination with SOC.
TruScient® study design and baseline characteristics
|
SOC |
TruScient® + SOC |
| Intervention |
Anaesthesia, perioperative pain management and fracture reduction / stabilisation as determined by vetinary surgeon |
TruScient® (rhBMP-2, 0.2 mg/mL in <2 sponges) implanted at fracture site and surgical protocol as in SOC group |
| N |
46 |
87 |
| Mean age (years) |
3.0 (range 0.6-13.0) |
3.8 (range 0.7-13.0) |
| Mean body weight (kg) |
17.1 (range 2.0-52.2) |
18.8 (range 1.7-70.9) |
| Primary Endpoint |
Time to radiographic fracture union ? |
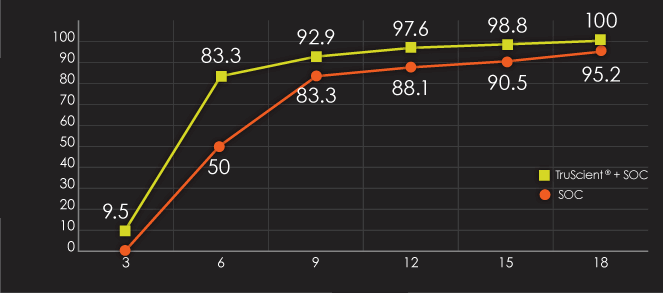
Radiographic fracture healing score improved over time for all dogs. However, the analysis demonstrated a statistically significant (open/closed; p=0.0001) reduction in the time to radiographic fracture union in the TruScient® + SOC vs SOC alone, regardless of fracture type.
Radiographic fracture union by week and treatment*
 Cumluative dogs with
Cumluative dogs with
fracture union (%)
Week
* Invalid efficacy data from 7 dogs were excluded from this analysis
At Week 6, radiographic union was achieved in:
- 83.3% of dogs using TruScient® + SOC
- 50% of dogs using SOC alone
By the end of the study, all dogs using TruScient® + SOC achieved radiographic fracture union, whilst two dogs in the SOC group did not.
A new clinical finding was reported as an adverse event when it was either unexpected or more serious than expected for the initial type of injury and postoperative time point.
Increased swelling is anticipated with TruScient® due to the associated neovascular induction, and is typically firm, non-painful and non-erythematous. Over 3-18 weeks, swelling was more frequent with TruScient® + SOC vs SOC alone.
At 3 weeks, moderate swelling was seen in:
- 35.6% of dogs using TruScient® + SOC
- 13.0% of dogs using SOC alone
The most frequently reported adverse event with TruScient® was lameness, reported in 12.6% of dogs using TruScient® + SOC vs 6.5% using SOC alone (p=ns).
The SOC alone group reported a higher frequency of hardware complications (15.2% vs 6.9%) and osteomyelitis (4.3% vs 0%) vs TruScient® + SOC.